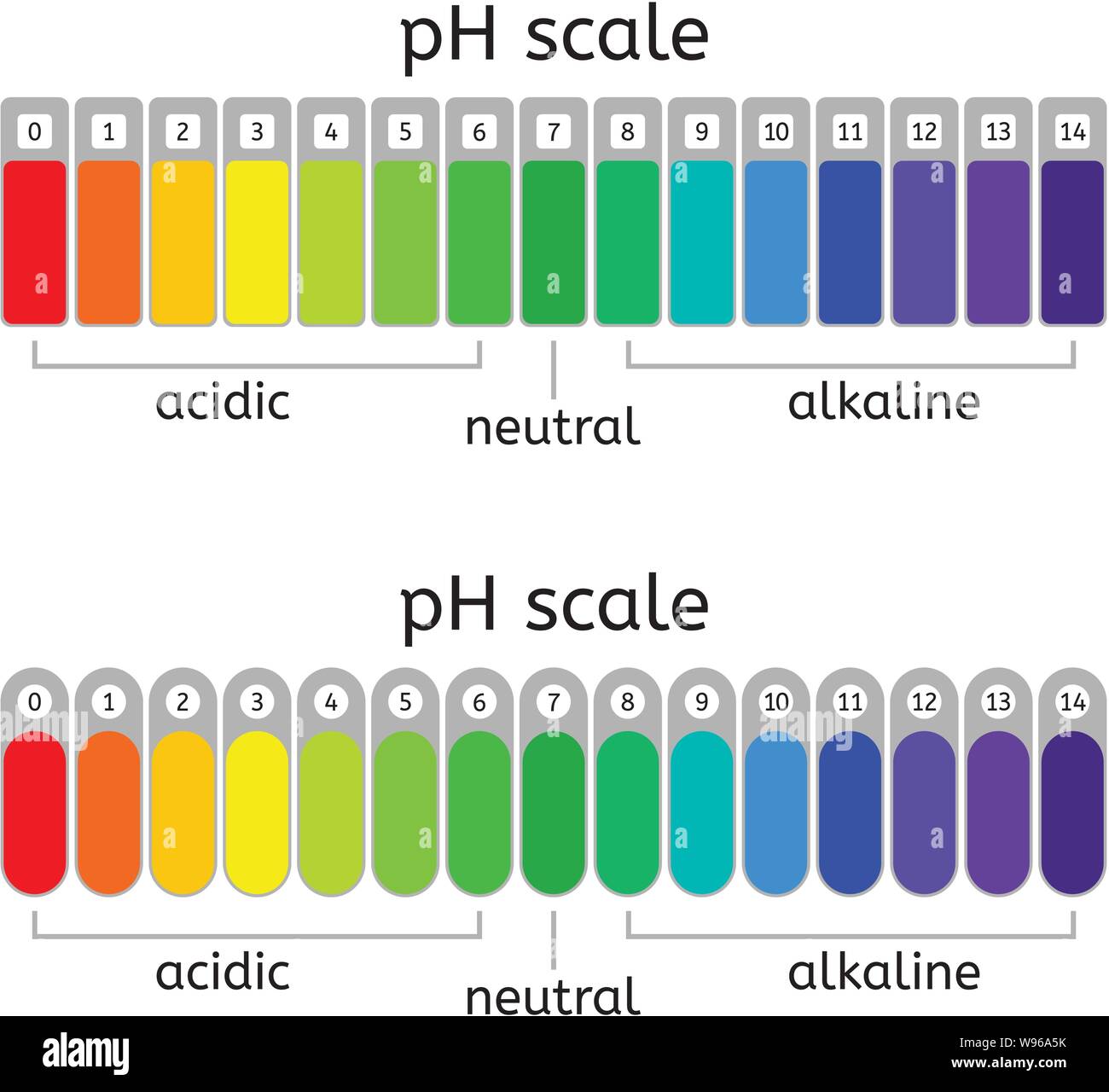
vector ph scale of acidic,neutral and alkaline value chart for acid and alkaline solutions. ph
Strictly speaking, pure water only has a pH of 7 at 'room temperature' (25˚C). Above and below this temperature, it can vary: for example, at 100˚C, the pH of pure water is 6.14, whilst at 0˚C, it's 7.47. This doesn't mean that the pure water is becoming acidic or alkaline, but that, at these temperatures, those particular pH numbers.
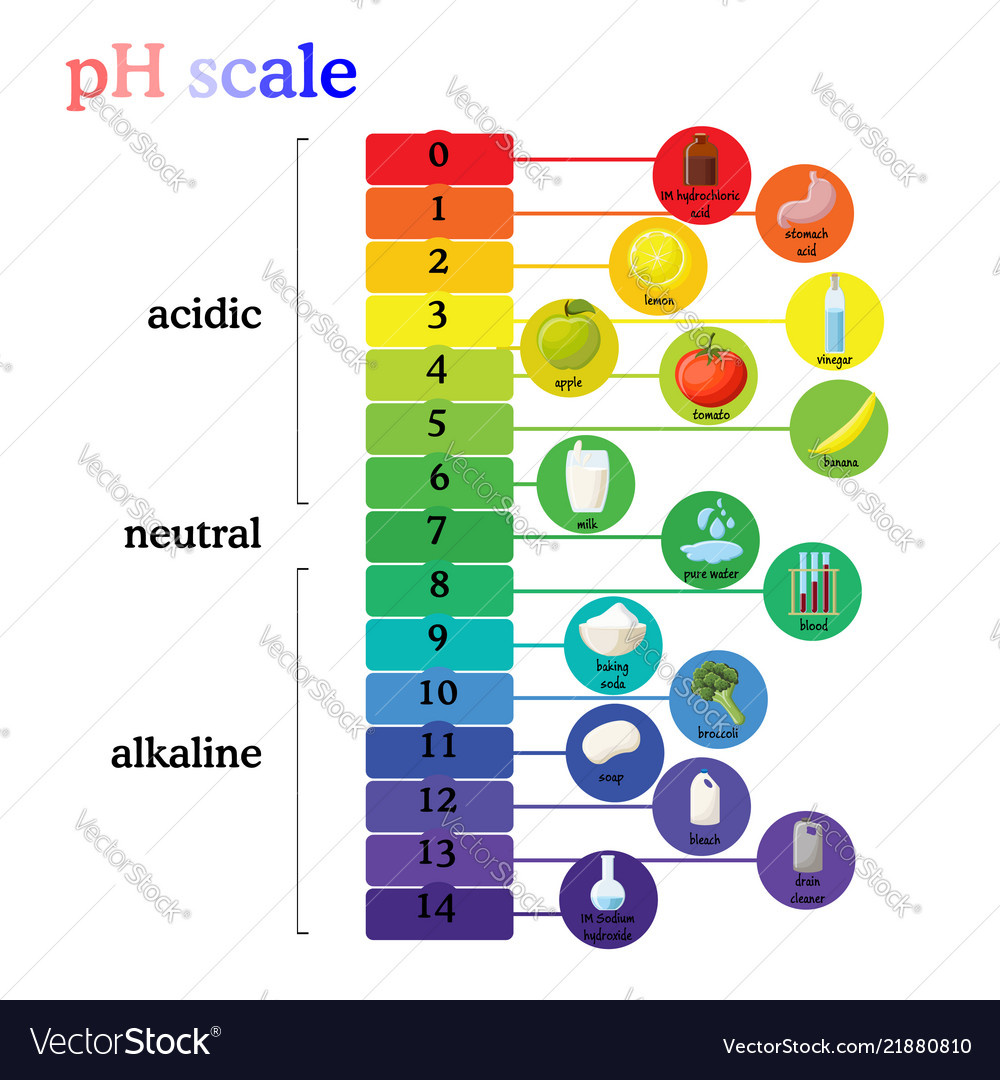
Ph scale diagram with corresponding acidic Vector Image
The pH scale is a number scale from 0 to 14. It tells us how acidic or alkaline an aqueous solution is. The pH scale is used to classify solutions as acidic, alkaline or neutral. Neutral.
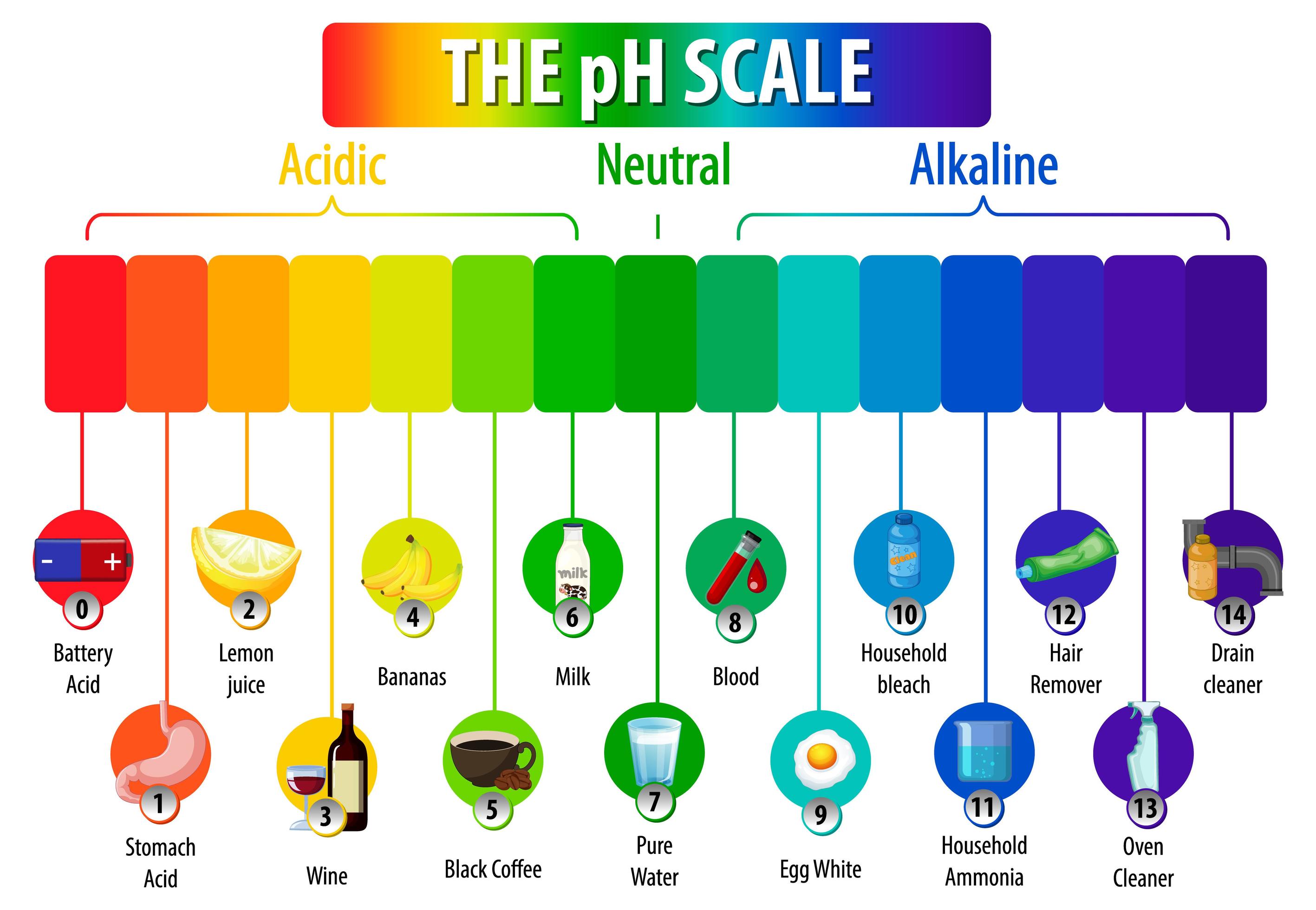
The pH Scale diagram on white background 1845080 Vector Art at Vecteezy
The pH scale is the range of value s from 0 to 14 that describes the acidity or basicity of a solution. You can use \(pH\) to make a quick determination whether a given aqueous solution is acidic, basic, or neutral.. The diagram below shows all of the interrelationships between [H3O+][H3O+], [OH−][OH−], pH, and pOH.
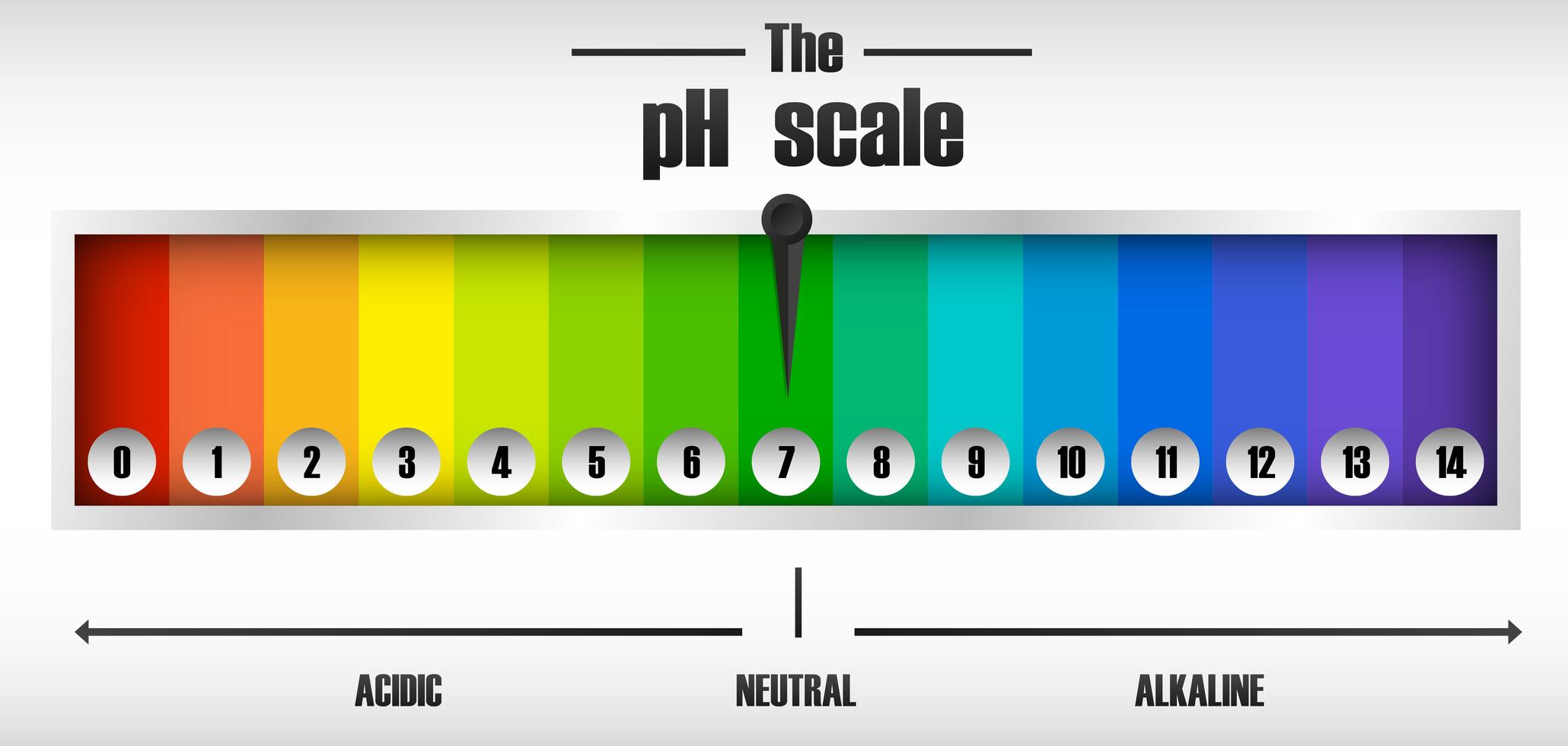
The ph scale diagram 589313 Vector Art at Vecteezy
pH Scale - PhET Interactive Simulations
The pH Scale diagram on white background 2988621 Vector Art at Vecteezy
The pH scale is the range of value s from 0 to 14 that describes the acidity or basicity of a solution. You can use \(pH\) to make a quick determination whether a given aqueous solution is acidic, basic, or neutral.. The diagram below shows all of the interrelationships between [H3O+][H3O+], [OH−][OH−], pH, and pOH.
PH Scale Diagram with Corresponding Acidic or Alcaline Values. Universal PH Indicator Paper
To give you the short answer: An acidic solution has a high concentration of hydrogen ions (H + ), greater than that of pure water. A basic solution has a low H + concentration, less than that of pure water. To see where this definition comes from, let's look at the acid-base properties of water itself. Autoionization of water

Ph scale diagram on white background Royalty Free Vector
The pH scale ranges from 0 to 14. Let's see why it is up to 14 only and not more than 14. When acidic H3O+ and basic OH- combine, they form water. If we study the dissociation of water we can solve the mystery of 14. 2H2O ⇌ H3O+ + OH- Or H2O + H2O ⇌ H3O+ ++ OH-

Back to Basics Acids, Bases & the pH Scale Precision Laboratories
Google Classroom Definitions of pH, pOH, and the pH scale. Calculating the pH of a strong acid or base solution. The relationship between acid strength and the pH of a solution. Key points We can convert between [ H +] and pH using the following equations: pH = − log [ H +] [ H +] = 10 − pH We can convert between [ OH −] and pOH

Ph Scale Chart Vector Illustration Stock Illustration Download Image Now iStock
Download this stock image: Diagram of the pH scale with examples of acidic, neutral and alkaline substances. - G156N5 from Alamy's library of millions of high resolution stock photos, illustrations and vectors.

pH Scale newagenutrients
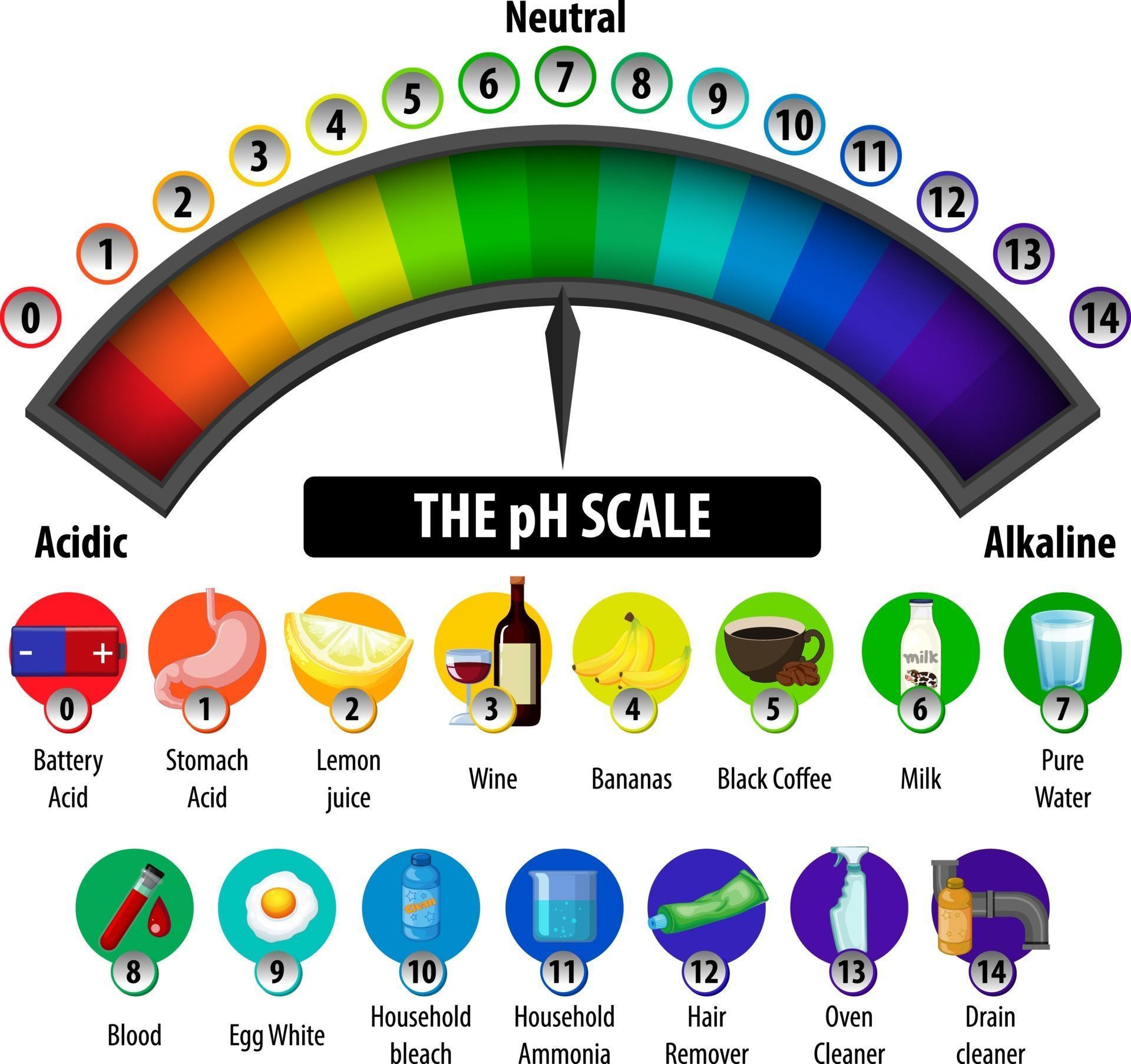
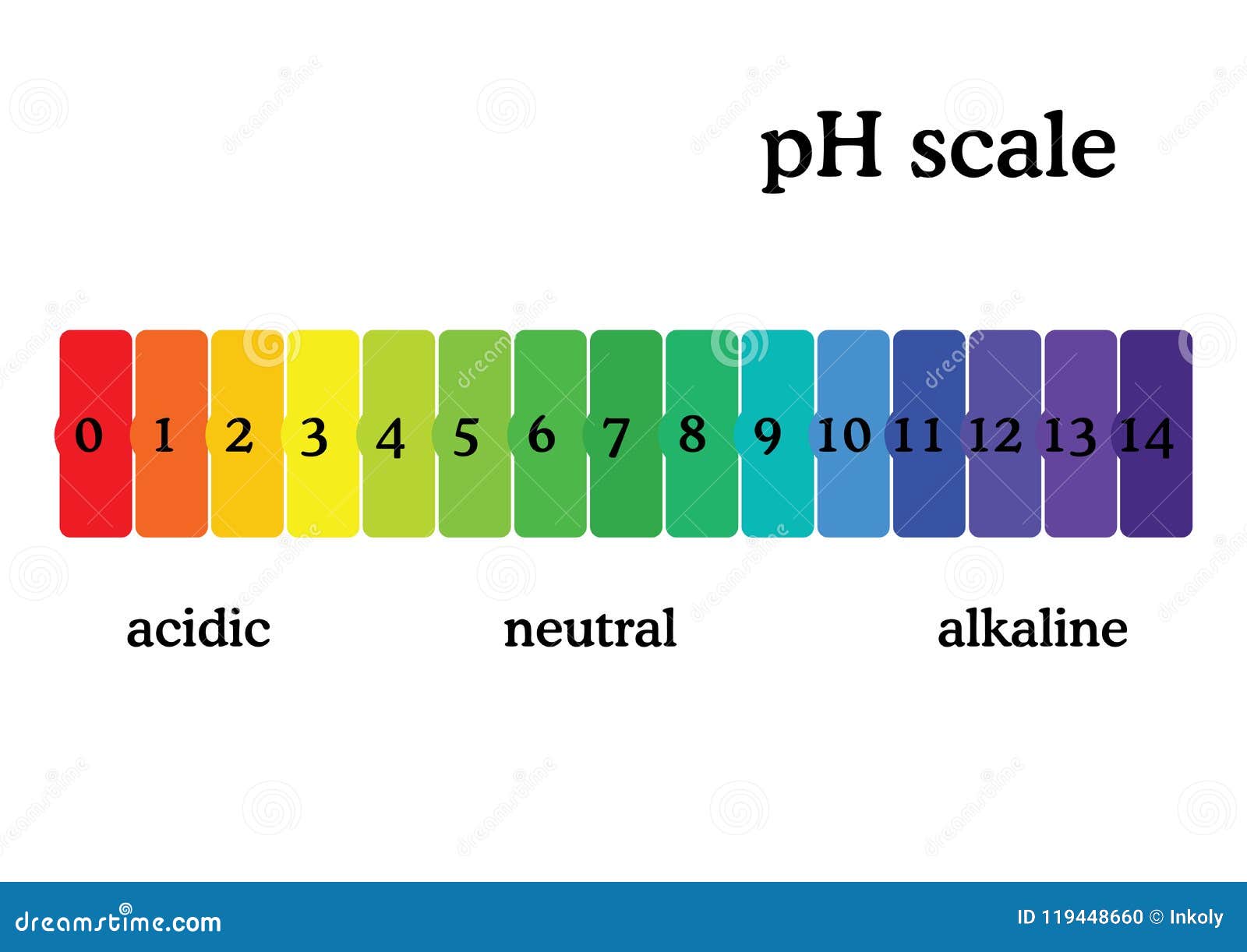
The pH scale is a commonly used scale to measure the acidity or the basicity of a substance. The possible values on the pH scale range from 0 to 14. Acidic substances have pH values ranging from 1 to 7 (1 being the most acidic point on the pH scale), and alkaline or basic substances have pH values ranging from 7 to 14.
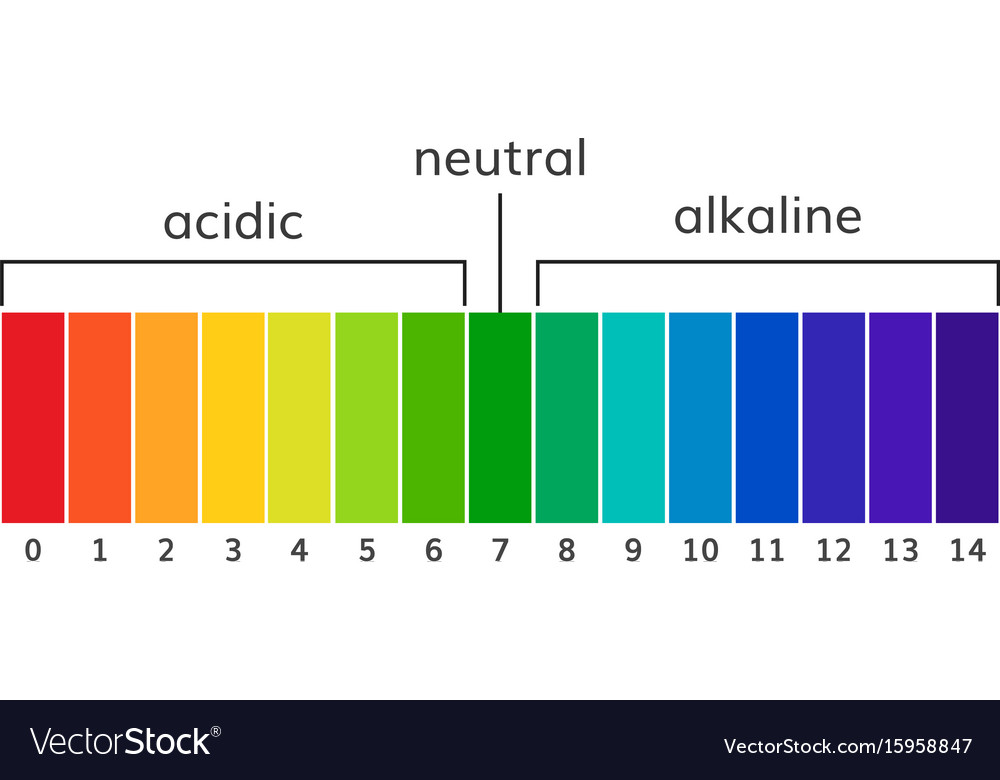
Chart ph alkaline and acidic scale Royalty Free Vector Image
The pH scale shows how acidic or basic a chemical is in aqueous solution (mixed with water). The scale runs from 0 (most acidic) to 14 (most alkaline or basic), where 7 is neutral pH. Chemicals with pH values from 0 up to 7 are acids, those with a pH value of 7 are neutral, and those with pH values greater than 7 up to 14 are bases.

File2713 pH Scale01.jpg
The determination of pH is one of the most common process chemical measurements made today. This booklet. and the pH values assigned to them define the pH scale. The procedure by which pH val-. Figure 2-1 shows a simplified diagram of a pH cell. The cell consists of a measuring electrode, a refer-ence electrode, a temperature sensing.

The ph scale diagram 541433 Vector Art at Vecteezy
Solution. The neat and labeled diagram of the pH scale is as shown. The range of pH is from 0 to 14. pH = 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful?
The Ph scale universal Indicator ph Color Chart diagram. Vector illustration with ph scale Stock
The range of pH goes from 0 to 14. A value less than 7 indicates that water is acidic. A value greater than 7 indicates that water is alkaline. A value equal to 7 shows that water is neutral. Each whole pH value below 7 is ten times more acidic than the next higher value. For example, a pH of 5 is ten times more acidic than 6.

How Learning the pH Scale Can Create a More Balanced Diet Natural Bio Health
In chemistry, pH (/ p iː ˈ eɪ tʃ / pee-AYCH), also referred to as acidity or basicity, historically denotes "potential of hydrogen" (or "power of hydrogen"). It is a scale used to specify the acidity or basicity of an aqueous solution.Acidic solutions (solutions with higher concentrations of hydrogen (H +) ions) are measured to have lower pH values than basic or alkaline solutions.

Diagram of the pH scale with examples of acidic, neutral and alkaline substances Stock Photo Alamy
Knowing the dependence of \(pH\) on \([H^+]\), we can summarize as follows: If pH < 7.00, then the solution is acidic. If pH = 7.00, then the solution is neutral. If pH > 7.00, then the solution is basic. This is known as the \(pH\) scale. The pH scale is the range of value s from 0 to 14 that describes the acidity or basicity of a solution.